
Technology & Privacy, Tax & Budgets, Health Care & Wellness
ICYMI: Major Emerging Legislative Trends in 2025 (Webinar Recap)
April 8, 2025 | Liz Malm
December 18, 2024 | Mary Kate Barnauskas
-2a7af5-1200px.jpeg)
Key Takeaways:
With rising prescription drug costs a concern for consumers, state legislatures have taken an array of actions to contain costs. In recent years the establishment of Prescription Drug Affordability Boards (PDABs) has emerged as a strategy across the country. Eleven states have established a PDAB or a similar drug cost control entity. Of note, Ohio's prescription drug transparency and affordability advisory council is no longer active after its authorizing statute was amended in 2021, but topics related to the council’s previous work can be examined by the Joint Medicaid Oversight Committee.
PDAB authority varies across states but can include making policy recommendations, creating reports on prescription drug issues, setting prescription drug spending benchmarks, conducting affordability/cost reviews on eligible drugs, or setting upper payment limits (UPLs). PDABs in four states (Colorado, Maryland, Minnesota, and Washington) have the authority to set UPLs on prescription drugs they determine to be unaffordable after review. UPLs are limits on the amount that the purchaser or payer can pay for a prescription drug product. Currently, no states have implemented a UPL, but several state PDABs have made significant movement on the issue, including Maryland, Oregon, and Colorado. As existing PDABs work through the various aspects of UPLs, other states will be watching and assessing UPL impact, which could lead to other states considering similar measures.
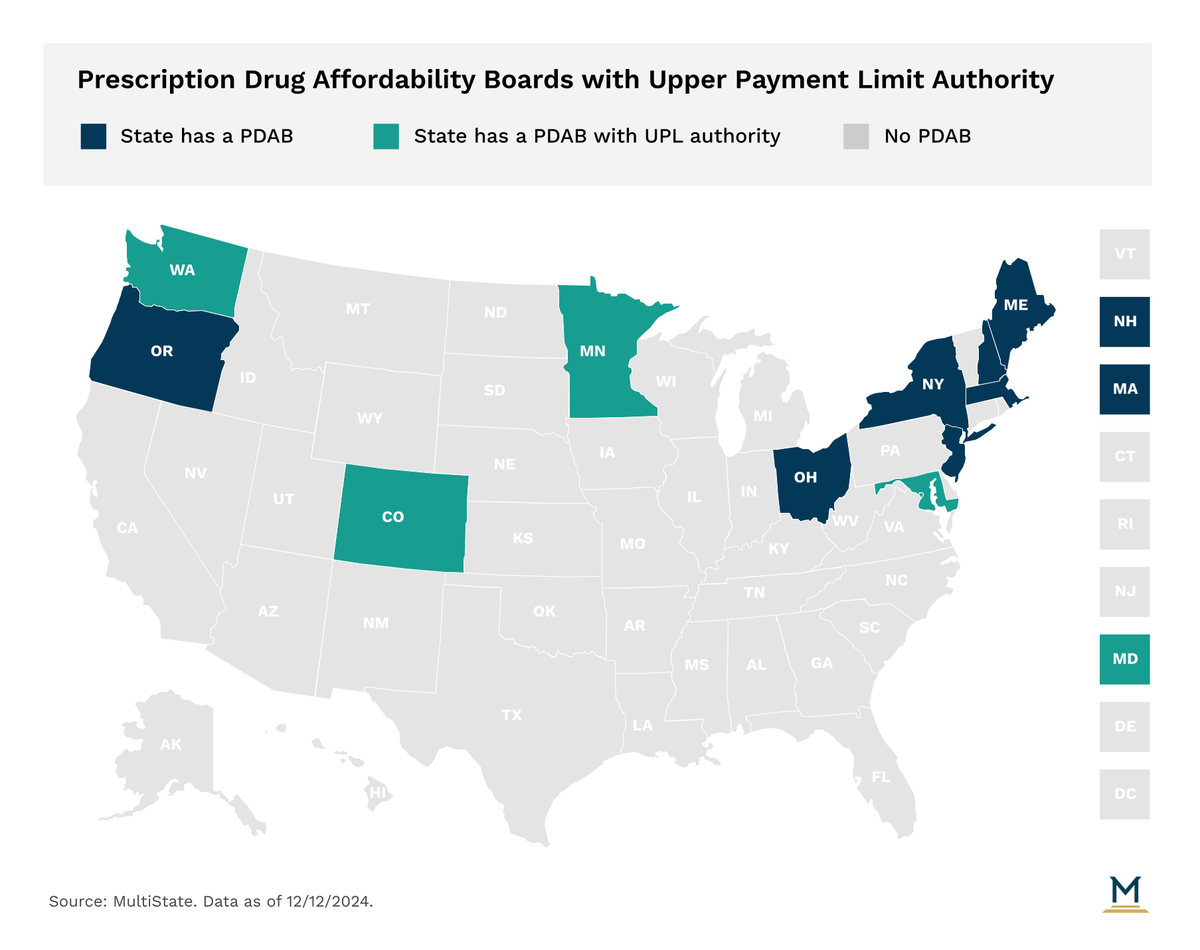
The Maryland PDAB is authorized by statute to set UPLs for public plans, but in order to exercise that authority it was required to develop an UPL Action Plan and get it approved by the General Assembly’s Legislative Policy Committee. In September, the PDAB approved the UPL Action Plan and in October the Legislative Policy Committee approved it. The process outlined in the report details how the PDAB will assess UPLs as a policy option for a prescription drug the PDAB has identified as having led or will lead to an affordability challenge during the cost review study process. The report also details the development of methodologies and calculations of UPL amounts. With the report approved, the PDAB is working through the rulemaking procedures to establish regulations to implement the process from the report. Earlier this year, the PDAB selected 6 drugs for cost review studies and is set to start the cost review process for the selected drugs next year.
The Oregon PDAB does not currently have UPL authority. However, in 2023 lawmakers enacted SB 192, which directed the PDAB to develop a plan for establishing UPLs in the state and provide a report on the plan to the legislature. Over the past year, the PDAB developed the report, and on November 20, 2024, the PDAB approved the final report. The report details potential approaches and methodologies for establishing UPLs, including net cost, reference pricing, launch price indexing, percentage off of WAC, value, and budget impact-based concepts. In addition, the report includes an analysis of potential cost savings, impact on stakeholders, enforcement, and implementation. The PDAB also included additional cost-saving solutions and complementary approaches to UPLs in the report, including pass-through pricing and value-based pricing. On December 11, the report was presented to the Senate Interim Committee on Health Care, and there will likely be continued discussion on the issue in the new session year.
The Colorado PDAB is the furthest along in the UPL process. The PDAB has completed affordability reviews on 5 drugs and determined that 3 of those drugs were unaffordable and selected them for UPLs. Over the past few months, the PDAB has been in discussions about UPL process implementation, data considerations, and stakeholder engagement. The PDAB will be setting UPLs for individual drugs through regulation and recently announced that they will be initiating the UPL rulemaking process for its first drug in January. Notably, Colorado’s PDAB is facing a lawsuit from a manufacturer with a drug selected for a UPL over the constitutionality of the PDAB and its processes. A lot of states will be watching Colorado to see how the UPL process plays out and the impact it has on affordability and the supply chain in the state, as well as for the outcome of the pending litigation, which will be consequential in determining the viability of PDABs as a policy solution to address drug affordability.
MultiState has an official PDABs monitoring apparatus, which brings you regular updates on the activity of PDABs along with intel on which states might establish additional PDABs. Our health policy team covers all PDAB and advisory council meetings and sends summaries of key decisions and discussions directly to your inbox. In addition to our reports, our team equips our client subscribers with timely information and actionable intelligence to make informed strategy decisions. For more information, reach out to our Health Care Policy Practice team.

April 8, 2025 | Liz Malm

April 2, 2025 | Townsend Brown

March 17, 2025 | Brock Ingmire